SinCellTE 2022
Single-cell Epigenomics & Multi-omics integration

Carl Herrmann & Andres Quintero
13 January 2022Chromatin accessibility scATAC-seq analysis with Signac
Data download
In this tutorial we will use the scRNA-seq/scATAC-seq multiome example data provided by 10x Genomics for human PBMCs. However, we will focus only on the scATAC-seq data, in the second session we will see how can we use the scRNA-seq data to improve our analysis.
The data was downloaded using the following commands:
Do not run!
wget https://cf.10xgenomics.com/samples/cell-arc/1.0.0/pbmc_granulocyte_sorted_10k/pbmc_granulocyte_sorted_10k_filtered_feature_bc_matrix.h5
wget https://cf.10xgenomics.com/samples/cell-arc/1.0.0/pbmc_granulocyte_sorted_10k/pbmc_granulocyte_sorted_10k_atac_fragments.tsv.gz
wget https://cf.10xgenomics.com/samples/cell-arc/1.0.0/pbmc_granulocyte_sorted_10k/pbmc_granulocyte_sorted_10k_atac_fragments.tsv.gz.tbi
Load the chromatin accessibility data
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
## Load packages ##
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
library(Signac)
library(Seurat)
library(EnsDb.Hsapiens.v86)
library(BSgenome.Hsapiens.UCSC.hg38)
# Setting up working directory
work_dir <- paste0("/shared/projects/sincellte_2022/", Sys.getenv('USER'), "/Single-cell_ATAC_analysis/results")
data_dir <- "/shared/projects/sincellte_2022/Courses/Single-cell_ATAC_analysis/input/data/"
dir.create(work_dir, recursive = TRUE)
setwd(work_dir)
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
## Load data ##
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
# load the RNA and ATAC data
counts <- Read10X_h5(paste0(data_dir, "pbmc_granulocyte_sorted_10k_filtered_feature_bc_matrix.h5"))
# If the previous line fails, please use the following:
# counts <- readRDS(paste0(data_dir, "pbmc_granulocyte_sorted_10k_filtered_feature_bc_matrix.RDS"))
fragments_files <- paste0(data_dir, "pbmc_granulocyte_sorted_10k_atac_fragments.tsv.gz")
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
## Load annotation ##
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
# get gene annotations for hg38
annotation <- GetGRangesFromEnsDb(ensdb = EnsDb.Hsapiens.v86)
seqlevelsStyle(annotation) <- "UCSC"
# If the previous line fails, please use the following:
# seqlevels(annotation) <- stringr::str_replace(
# string = paste("chr", seqlevels(annotation),sep=""),
# pattern = "chrMT", replacement="chrM")
# Check annotation seqnames
unique(seqnames(annotation))
# Check counts
lapply(counts, dim)
lapply(counts, class)
Click for Answer
> unique(seqnames(annotation))
[1] chrX chr20 chr1 chr6 chr3 chr7 chr12 chr11 chr4 chr17 chr2 chr16 chr8 chr19 chr9 chr13 chr14 chr5 chr22 chr10
[21] chrY chr18 chr15 chr21 chrM
25 Levels: chrX chr20 chr1 chr6 chr3 chr7 chr12 chr11 chr4 chr17 chr2 chr16 chr8 chr19 chr9 chr13 chr14 chr5 ... chrM
> lapply(counts, dim)
$`Gene Expression`
[1] 36601 11909
$Peaks
[1] 108377 11909
> lapply(counts, class)
$`Gene Expression`
[1] "dgCMatrix"
attr(,"package")
[1] "Matrix"
$Peaks
[1] "dgCMatrix"
attr(,"package")
[1] "Matrix"
Create a Signac object from the Cellranger peak counts
To create a Signac object, the first step is to create a Chromatin Assay object using the Cellranger peak counts, and then create a Seurat object from this:
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
## Create Signac ##
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
# Create a ChromatinAssay
chrom_assay <- CreateChromatinAssay(
counts = counts$Peaks,
sep = c(":", "-"),
fragments = fragments_files,
min.cells = 10,
min.features = 200
)
# Create a Seurat object from the ChromatinAssay
signacobj <- CreateSeuratObject(
counts = chrom_assay,
assay = "ATAC"
)
rm(chrom_assay, counts)
gc()
# Add the gene information to the object, this allows downstream functions to
# pull the gene annotation information directly from the object.
Annotation(signacobj) <- annotation
signacobj
Click for Answer
An object of class Seurat
106086 features across 11830 samples within 1 assay
Active assay: ATAC (106086 features, 0 variable features)
Signac adds extra functionality to a Seurat object, for instance a genomic ranges associated with each feature in the object can be retreived when the active assay is a ChromatinAssay.
granges(signacobj)
Click for Answer
GRanges object with 106086 ranges and 0 metadata columns:
seqnames ranges strand
<Rle> <IRanges> <Rle>
[1] chr1 10109-10357 *
[2] chr1 180730-181630 *
[3] chr1 191491-191736 *
[4] chr1 267816-268196 *
[5] chr1 586028-586373 *
... ... ... ...
[106082] KI270713.1 20444-22615 *
[106083] KI270713.1 27118-28927 *
[106084] KI270713.1 29485-30706 *
[106085] KI270713.1 31511-32072 *
[106086] KI270713.1 37129-37638 *
-------
seqinfo: 33 sequences from an unspecified genome; no seqlengths
Quality control
The following are some metrics and threshold ranges recommended by the developers of Signac (the final values always depend on the biological system, and experimental conditions):
-
Nucleosome banding pattern: the histogram of DNA fragment sizes (determined from the paired-end sequencing reads) should exhibit a strong nucleosome banding pattern corresponding to the length of DNA wrapped around a single nucleosome. We calculate this per single cell, and quantify the approximate ratio of mononucleosomal to nucleosome-free fragments (stored as nucleosome_signal)
-
Transcriptional start site (TSS) enrichment score: the ENCODE project has defined an ATAC-seq targeting score based on the ratio of fragments centered at the TSS to fragments in TSS-flanking regions (see https://www.encodeproject.org/data-standards/terms/). Poor ATAC-seq experiments typically will have a low TSS enrichment score. We can compute this metric for each cell with the TSSEnrichment() function, and the results are stored in metadata under the column name
TSS.enrichment -
Total number of fragments: A measure of cellular sequencing depth / complexity. Cells with very few reads may need to be excluded due to low sequencing depth. Cells with extremely high levels may represent doublets, nuclei clumps, or other artefacts.
-
Fraction of fragments in peaks: Represents the fraction of all fragments that fall within ATAC-seq peaks. Cells with low values (i.e. <15-20%) often represent low-quality cells or technical artifacts that should be removed. Note that this value can be sensitive to the set of peaks used.
-
Ratio reads in genomic blacklist regions: The ENCODE project has provided a list of blacklist regions, representing reads which are often associated with artefactual signal. Cells with a high proportion of reads mapping to these areas (compared to reads mapping to peaks) often represent technical artifacts and should be removed. ENCODE blacklist regions for human (hg19 and GRCh38), mouse (mm10), Drosophila (dm3), and C. elegans (ce10) are included in the Signac package.
Taken from https://satijalab.org/signac/articles/pbmc_vignette.html_
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
## QC ##
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
# compute nucleosome signal score per cell
signacobj <- NucleosomeSignal(signacobj)
# compute TSS enrichment score per cell
signacobj <- TSSEnrichment(signacobj, fast = FALSE)
# Counting fraction of reads in peaks
total_fragments <- CountFragments(fragments_files)
signacobj$fragments <- total_fragments$reads_count[match(colnames(signacobj),
total_fragments$CB)]
signacobj <- FRiP(
object = signacobj,
assay = "ATAC",
total.fragments = "fragments"
)
# Counting fragments in genome blacklist regions
signacobj$blacklist_fraction <- FractionCountsInRegion(
object = signacobj,
assay = "ATAC",
regions = blacklist_hg38_unified
)
# Inspect the TSS enrichment scores by plotting the accessibility signal over all TSS sites.
signacobj$high.tss <- ifelse(signacobj$TSS.enrichment > 2, 'High', 'Low')
TSSPlot(signacobj, group.by = 'high.tss') + NoLegend()
Click for Answer
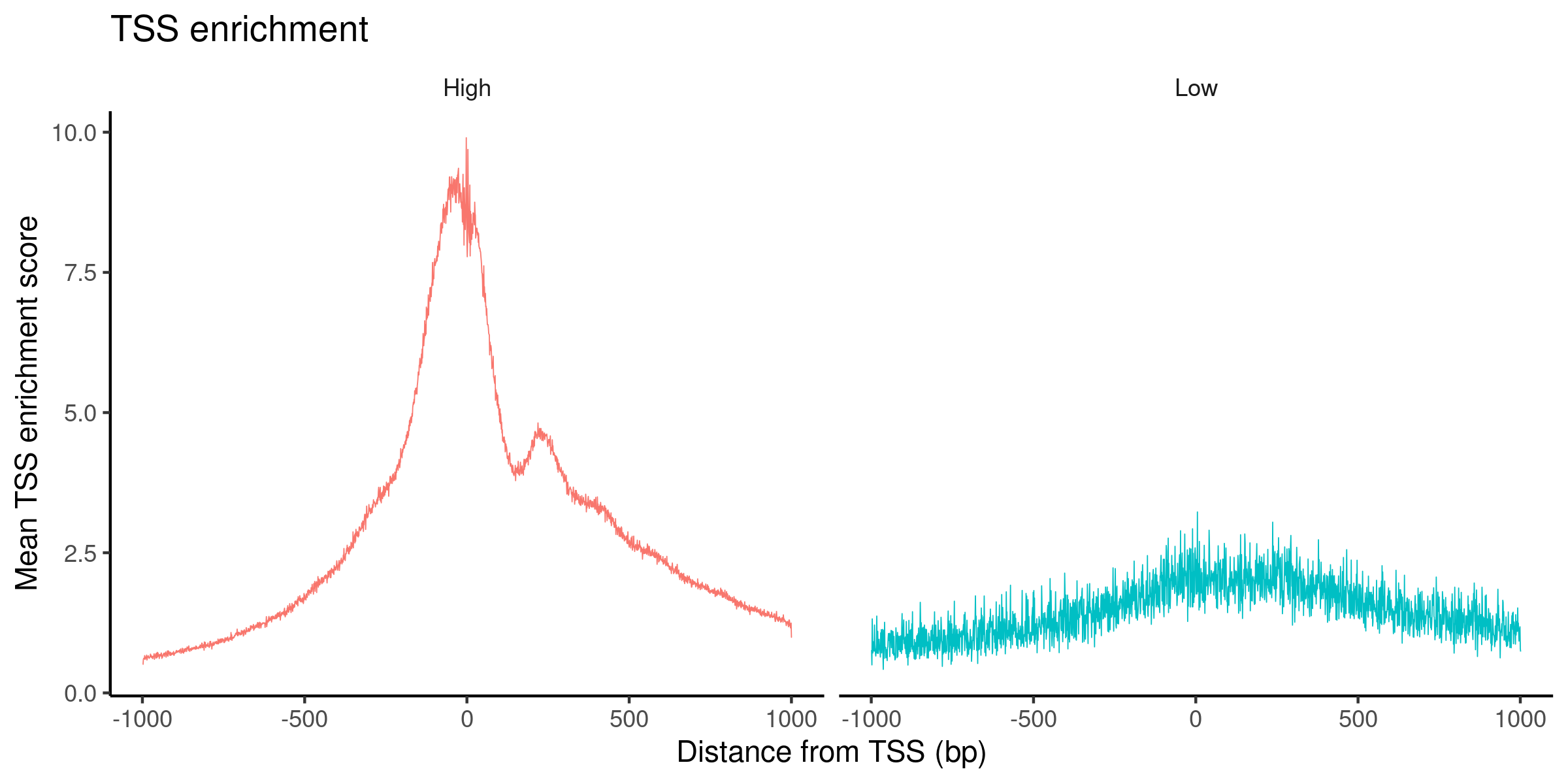
We can now visualize all the QC metrics and filter out all the low quality cells:
VlnPlot(
object = signacobj,
features = c("FRiP", "nCount_ATAC", "TSS.enrichment", "blacklist_fraction", "nucleosome_signal"),
ncol = 5,
pt.size = 0.1
)
# filter out low quality cells
signacobj <- subset(
x = signacobj,
subset =
nCount_ATAC < 100000 &
nCount_ATAC > 1000 &
FRiP > 0.15 &
blacklist_fraction < 0.01 &
nucleosome_signal < 2 &
TSS.enrichment > 1
)
signacobj
Click for Answer
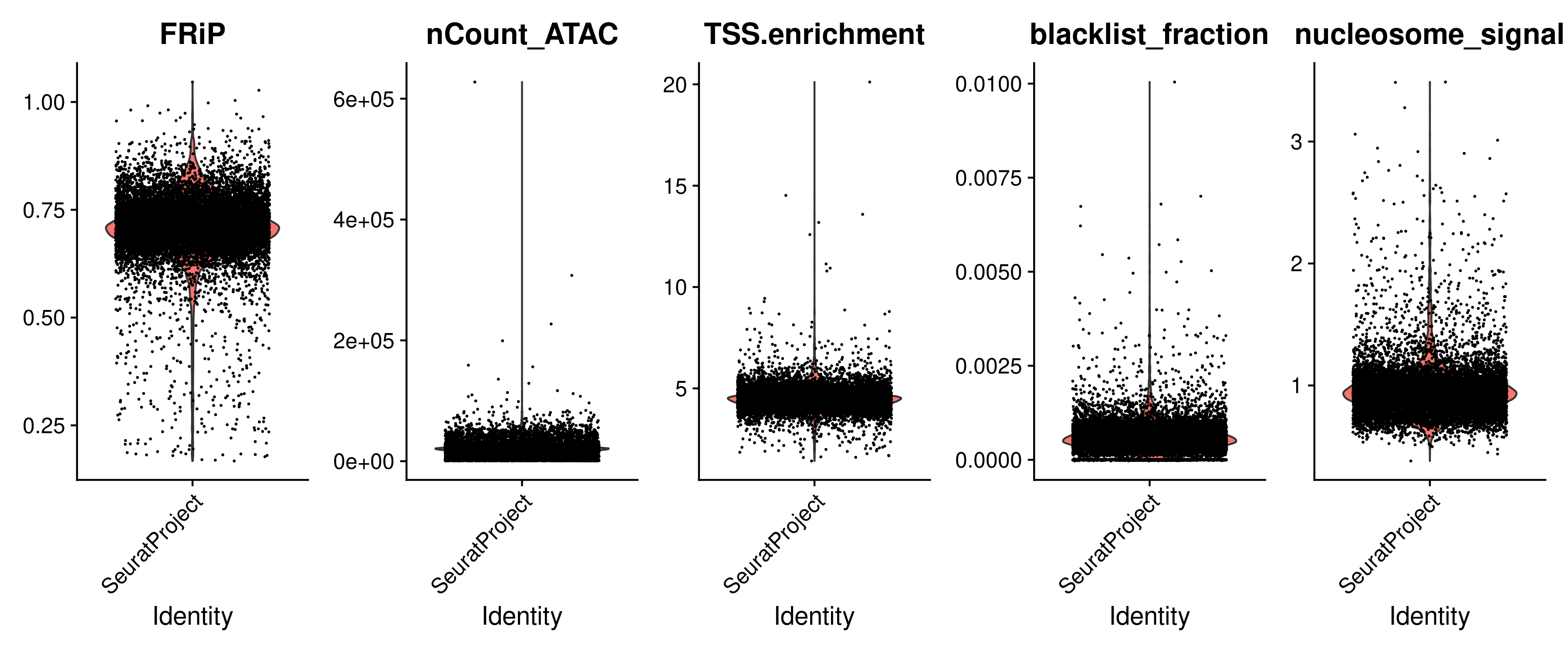
An object of class Seurat
106086 features across 11498 samples within 1 assay
Active assay: ATAC (106086 features, 0 variable features)
Checkpoint 1
Key questions:
- What is the FRiP?
- Why do we blacklist some genomic regions?
- How does the TSS enrichment score help us to remove low quality cells?
- Is there a consensus set of thresholds to identify low quality cells?
Call peaks and add peak counts matrix
The set of peaks identified using Cellranger often merges distinct peaks that are close together. This can create a problem for certain analyses, particularly motif enrichment analysis and peak-to-gene linkage. To identify a more accurate set of peaks, we can call peaks using MACS2 with the CallPeaks() function (Taken from: https://satijalab.org/signac/articles/pbmc_multiomic.html#linking-peaks-to-genes).
Do not run the next chunk!!!, as calling peaks and creating the feature matrix can take a long time. Instead we will read a precomputed feature matrix.
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
## Call peaks and make feature matrix ##
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
# Call peaks using MACS2 ~10min
peaks <- CallPeaks(signacobj, macs2.path = "/shared/software/miniconda/envs/macs2-2.2.7.1/bin/macs2")
# remove peaks on nonstandard chromosomes and in genomic blacklist regions
peaks <- keepStandardChromosomes(peaks, pruning.mode = "coarse")
peaks <- subsetByOverlaps(x = peaks, ranges = blacklist_hg38_unified, invert = TRUE)
# quantify counts in each peak ~15min
macs2_counts <- FeatureMatrix(
fragments = Fragments(signacobj),
features = peaks,
cells = colnames(signacobj)
)
Load precompute feature matrix and add to the seurat object:
# Read feature matrix and peaks
peaks <- readRDS(paste0(data_dir, "signac_peaks.RDS"))
macs2_counts <- readRDS(paste0(data_dir, "signac_macs2_counts.RDS"))
signacobj <- signacobj[,colnames(signacobj) %in% colnames(macs2_counts)]
macs2_counts <- macs2_counts[,colnames(signacobj)]
# create a new assay using the MACS2 peak set and add it to the Seurat object
signacobj[["peaks"]] <- CreateChromatinAssay(
counts = macs2_counts,
fragments = fragments_files,
annotation = annotation
)
peaks
Click for Answer
GRanges object with 131364 ranges and 6 metadata columns:
seqnames ranges strand | name score fold_change
<Rle> <IRanges> <Rle> | <character> <integer> <numeric>
[1] chr1 10032-10322 * | SeuratProject_peak_44 142 4.99234
[2] chr1 180709-181030 * | SeuratProject_peak_45 149 5.12372
[3] chr1 181296-181600 * | SeuratProject_peak_46 291 7.35714
[4] chr1 191304-191914 * | SeuratProject_peak_47 142 4.99234
[5] chr1 267874-268087 * | SeuratProject_peak_48 134 4.86097
... ... ... ... . ... ... ...
[131360] chrX 155880631-155881911 * | SeuratProject_peak_1.. 824 8.67288
[131361] chrX 155891339-155891781 * | SeuratProject_peak_1.. 105 4.30809
[131362] chrX 155966929-155967163 * | SeuratProject_peak_1.. 134 4.86097
[131363] chrX 155997247-155997787 * | SeuratProject_peak_1.. 263 6.17155
[131364] chrX 156029849-156030260 * | SeuratProject_peak_1.. 106 4.33546
neg_log10pvalue_summit neg_log10qvalue_summit relative_summit_position
<numeric> <numeric> <integer>
[1] 16.3379 14.2177 126
[2] 17.1108 14.9684 124
[3] 31.7325 29.1857 137
[4] 16.3379 14.2177 145
[5] 15.5761 13.4782 134
... ... ... ...
[131360] 87.4204 82.4206 637
[131361] 12.5625 10.5583 258
[131362] 15.5761 13.4782 112
[131363] 28.7770 26.3134 342
[131364] 12.6467 10.6377 245
-------
seqinfo: 24 sequences from an unspecified genome; no seqlengths
Reduce data dimensionality with LSI
Signac uses latent semantic indexing (LSI) to reduce the data dimensionality as well as ArchR, which is the combination of term frequency-inverse document frequency (TF-IDF) normalization followed by singular value decomposition (SVD).
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
## Normalize ATAC data ##
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
DefaultAssay(signacobj) <- "peaks"
signacobj <- FindTopFeatures(signacobj, min.cutoff = "q75")
signacobj <- RunTFIDF(signacobj)
signacobj <- RunSVD(signacobj, n=20)
# The first LSI component often captures sequencing depth (technical variation)
# rather than biological variation. If this is the case, the component should
# be removed from downstream analysis. We can assess the correlation between
# each LSI component and sequencing depth using the DepthCor() function:
DepthCor(signacobj)
Click for Answer
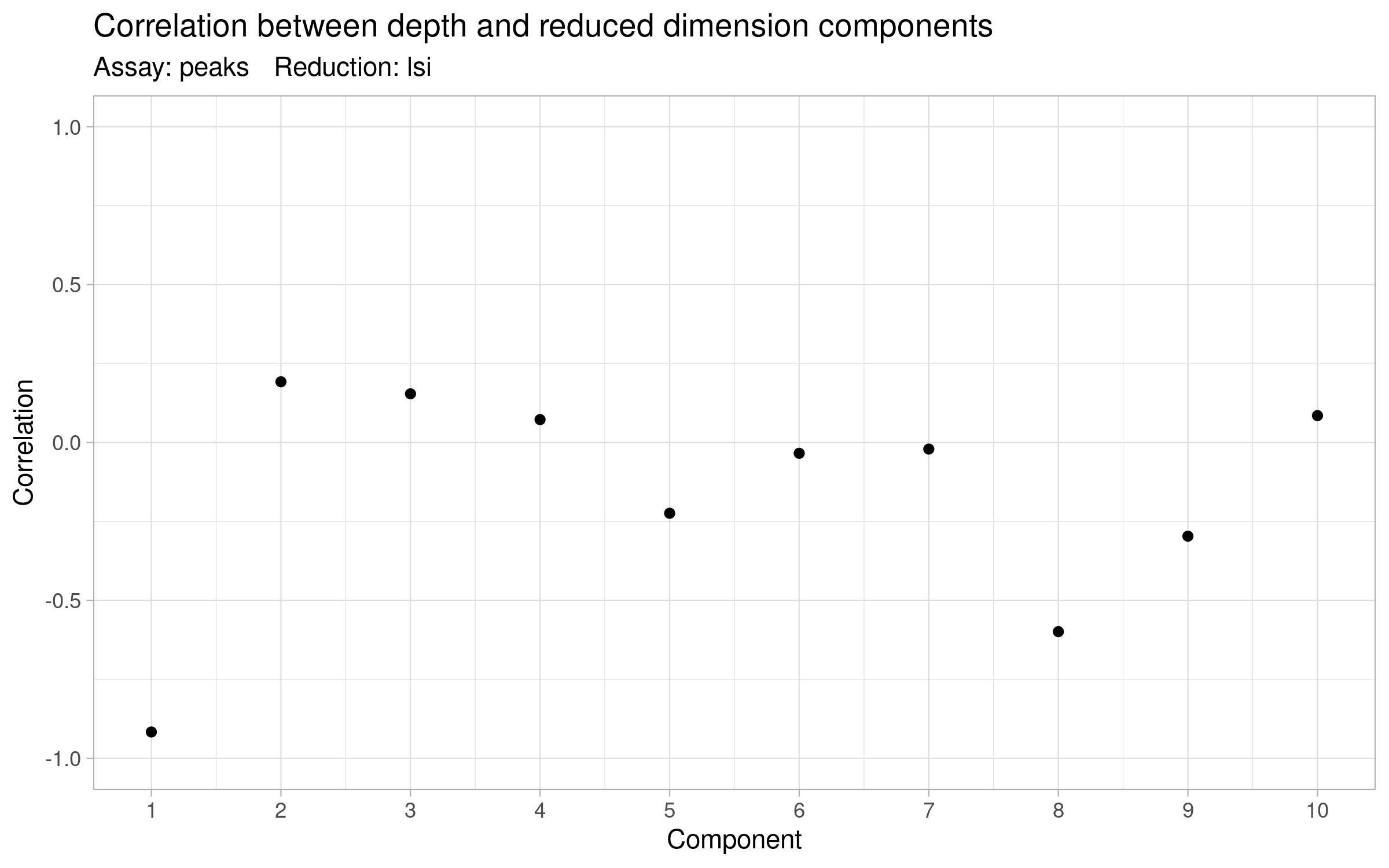
Then we use the LSI results to perform UMAP and find clusters of cells:
signacobj <- RunUMAP(object = signacobj, reduction = 'lsi', dims = 2:20)
signacobj <- FindNeighbors(object = signacobj, reduction = 'lsi', dims = 2:20)
signacobj <- FindClusters(object = signacobj, verbose = FALSE, algorithm = 3)
DimPlot(object = signacobj, label = TRUE) + NoLegend()
Click for Answer
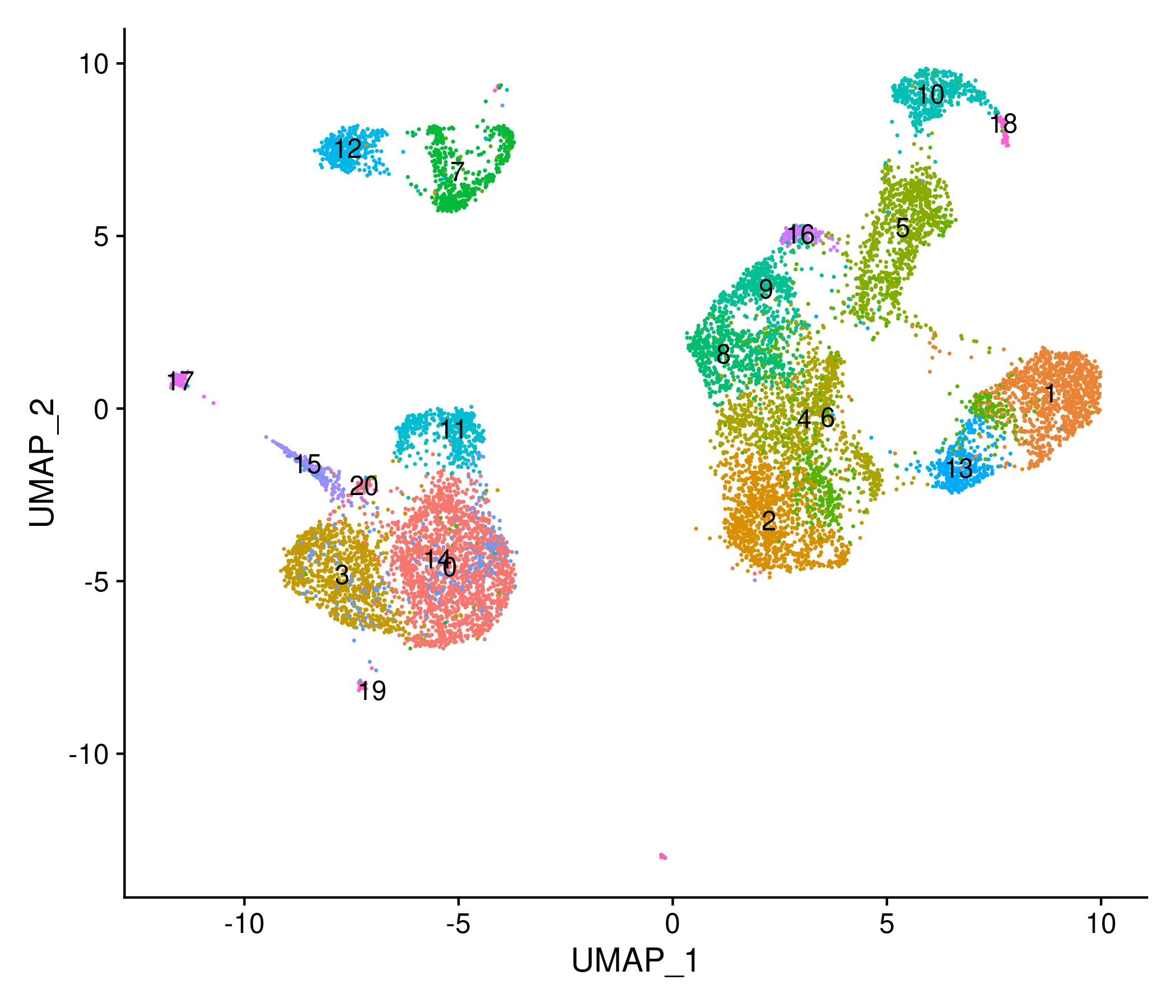
Checkpoint 2
Key questions:
- How does the min.cutoff parameter from
FindTopFeaturesaffects the final embedding? - What is the difference between Signac's
FindTopFeaturesand SeuratFindVariableFeatures? - How does the n parameter from
RunSVDaffects the final embedding?
Computing a gene activity matrix
The activity of each gene can be measured from the scATAC-seq data by quantifying the chromatin accessibility associated with each gene. In the case of Signac the gene activity matrix if computed by the following steps:
- Extract gene coordinates and extend them to include the 2 kb upstream region (promoter region).
- Count the number of fragments for each cell that map to each of these regions.
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
## Gene activity matrix ##
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
# gene_activities <- GeneActivity(signacobj) # Don't run! read percomputed object ~8min
gene_activities <- readRDS(paste0(data_dir, "signac_gene_activities.RDS"))
# add the gene activity matrix to the Seurat object as a new assay and normalize it
head(signacobj@meta.data)
signacobj[['RNA']] <- CreateAssayObject(counts = gene_activities)
signacobj <- NormalizeData(
object = signacobj,
assay = 'RNA',
normalization.method = 'LogNormalize',
scale.factor = median(signacobj$nCount_RNA)
)
The gene activities can be used to visualize the expression of marker genes on the scATAC-seq clusters:
DefaultAssay(signacobj) <- 'RNA'
FeaturePlot(
object = signacobj,
features = c('MS4A1', 'CD3D', 'LEF1', 'NKG7', 'TREM1', 'LYZ'),
pt.size = 0.1,
max.cutoff = 'q95',
ncol = 3
)
Click for Answer
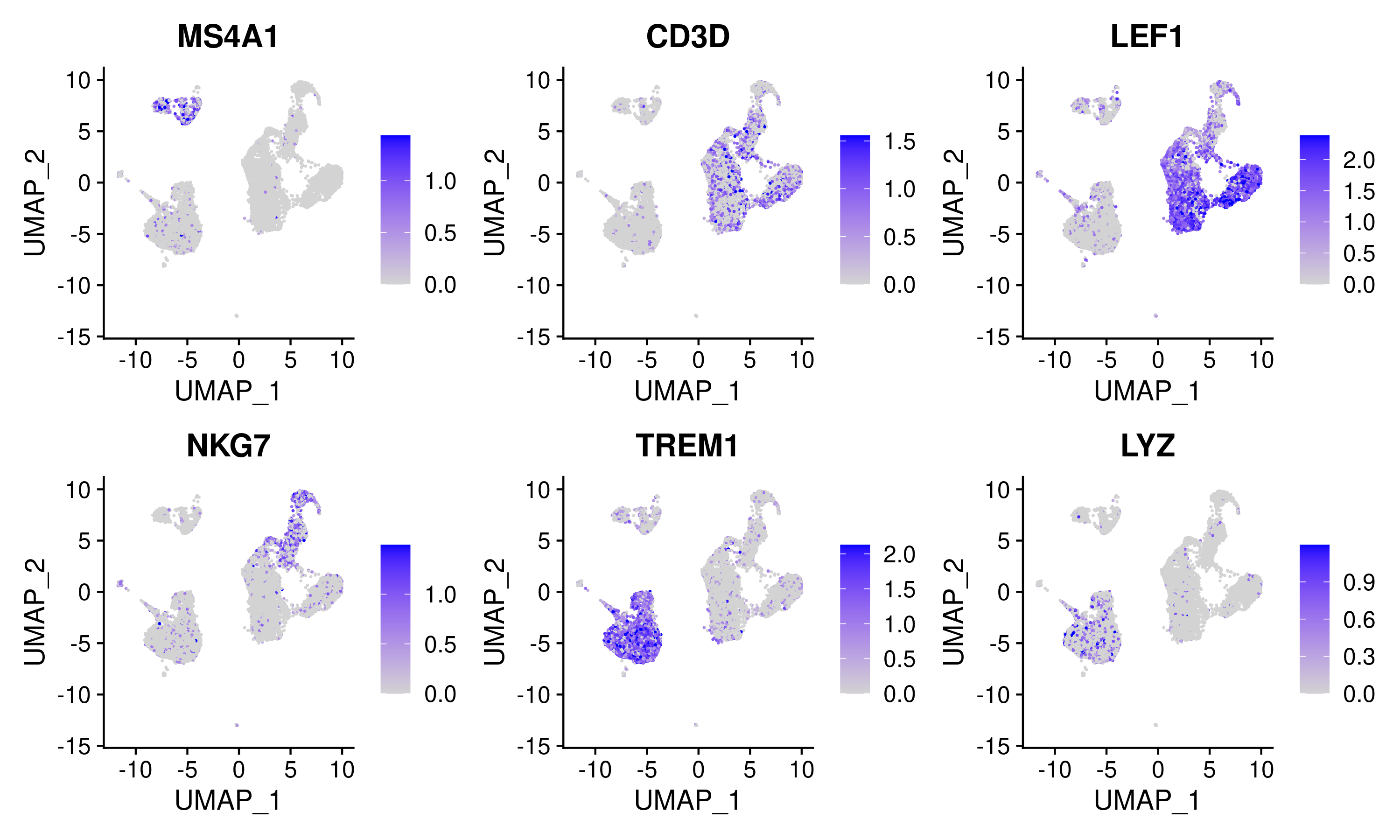
Annotating cell types with a reference dataset
We can use a reference scRNA-seq dataset to annotate cells from a scATAC-seq dataset. In this example, we will use different functions from the Seurat package for this. As a reference, we will use a pre-processed scRNA-seq dataset for human PBMCs. Provided by 10x Genomics, and pre-processed by the Satija Lab.
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
## Cell type annotation ##
##––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––##
reference <- readRDS(paste0(data_dir, "pbmc_10k_v3.rds"))
transfer_anchors <- FindTransferAnchors(
reference = reference,
query = signacobj,
reduction = 'cca'
)
predicted_labels <- TransferData(
anchorset = transfer_anchors,
refdata = reference$celltype,
weight.reduction = signacobj[['lsi']],
dims = 2:20
)
signacobj <- AddMetaData(object = signacobj, metadata = predicted_labels)
# set the cell identities to the cell type predictions
Idents(signacobj) <- "predicted.id"
# set a reasonable order for cell types to be displayed when plotting
levels(signacobj) <- c("CD4 Naive", "CD4 Memory", "CD8 Naive", "CD8 effector",
"Double negative T cell", "NK dim", "NK bright",
"B cell progenitor", "pre-B cell", "CD14+ Monocytes",
"CD16+ Monocytes", "pDC", "Dendritic cell", "Platelet")
DimPlot(
object = signacobj,
group.by = "predicted.id",
label = TRUE,
repel = TRUE) + NoLegend()
Click for Answer
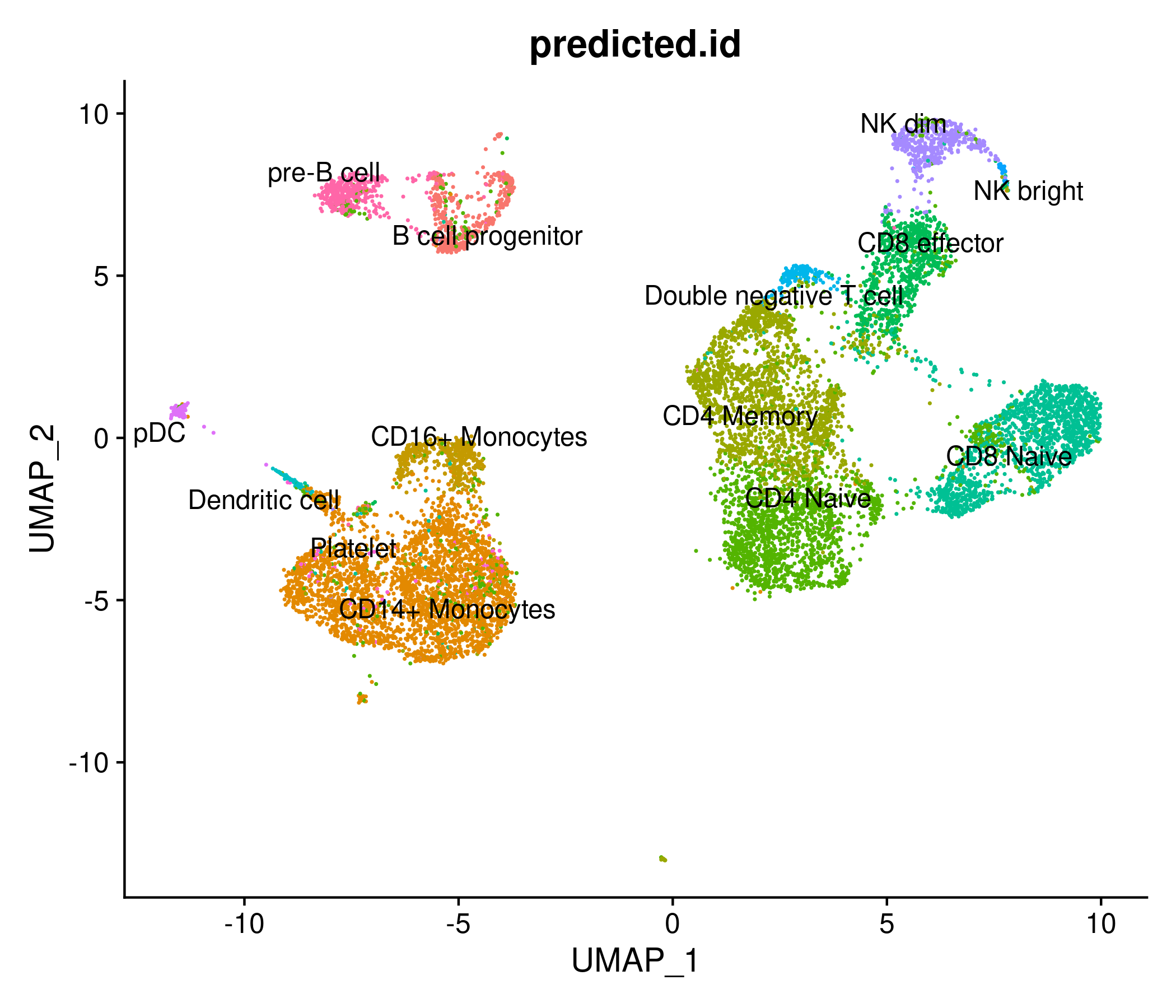
Checkpoint 3
Key questions:
- What happen if you use less dimensions (dims) in the function
TransferData - Which matrix is used by Signac's
FindTransferAnchorsbetween a scRNA-seq reference and a scATAC-seq query?